Agriculture
The constant exchange of a mineral or elemental nutrient between organisms and the physical environment is called a biogeochemical cycle. Along with the carbon cycle and the oxygen cycle, one of the most important biogeochemical cycles is that of the element phosphorus.
The phosphorus cycle involves the movement of the element phosphorus as it circulates through the living and nonliving portions of the biosphere.
Many of the chemical elements found on the earth are vital to the processes and systems of living organisms. Unlike oxygen and carbon, phosphorus follows complex pathways. It circulates through the earth?s soils, rocks, waters, and atmosphere and through the organisms that inhabit these many ecosystems.
Elements or minerals are stored in discrete parts of the earth?s ecosystems called compartments. Examples of compartments include all the plants in a forest, a certain species of tree, or even the leaves or needles of a tree.
Chemical elements reside within the compartments in certain amounts, or pools. A basic description of biogeochemical cycles involves following nutrients in the form of minerals or elements from pool to pool through the multitudes of ecosystem compartments.
Phosphorus and Plants
Phosphorus compounds reside primarily in rocks. Phosphorus does not go through an atmospheric phase, but rather, phosphorus-laden rocks release phosphate (PO4?3) into the ecosystem as the result of weathering and erosion.
To plants, phosphorus is a vital nutrient (second only to nitrogen). Plants absorb phosphates through their root hairs. Phosphorus then passes on through the food chain when the plants are consumed by other organisms.
Phosphorus is an essential component of many biological molecules, including deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Adenosine triphosphate (ATP), one of the nucleotides that make up DNA and RNA, is also the main energy transfer molecule in the multitude of chemical reactions taking place within organisms.
Because phosphorus is a major plant nutrient, massive amounts of phosphate-based fertilizers are either derived from natural sources (in the form of bat or bird guano) or chemically manufactured for use by agriculture.
As late as the early 1970?s, phosphates were a major constituent of household detergents, until it was discovered that large amounts of phosphates were being released into the environment.
In aquatic systems such as rivers and lakes, where such runoff eventually appears, an infusion of phosphates can cause algal blooms (rapidly forming, dense populations of algae). When the algae die, they are consumed by bacteria.
Decomposition by bacteria requires large amounts of oxygen, which soon depletes the available oxygen in the water. If the process is allowed to continue unchecked, fish and other organisms die from lack of oxygen. Both phosphates and nitrates contribute to cultural eutrophication.
Phosphates not taken up by plants go into the sedimentary phase, where they are very chemically reactive with other minerals. Some of these reactions produce compounds that effectively remove phosphates from the active nutrient pool.
This sedimentary phase is characterized by its long residence time compared to the rapid cycling through the biological phase. Phosphates can remain locked up in rocks for millions of years before being exposed and broken down by weathering, which once again makes them available to plants.
Phosphorus and the Environment
Because the phosphorus cycle is so complex, its interactions with other biogeochemical cycles are not completely understood. The study of these interactions is emerging as a vital field among the environmental sciences.
Excessive phosphates in a eutrophic lake disrupt the carbon cycle by reacting with bicarbonates, thus increasing the pH. Many freshwater organisms depend on a neutral pH level for their survival.
The presence of phosphorus under these oxygen-depleted conditions can also indirectly affect the sulfur cycle, leading to the conversion of sulfate to sulfide. When sulfide combines with hydrogen to form the gas hydrogen sulfide, it takes on the familiar "rotten egg" smell.
One of the keys to preventing environmental degradation through the altering of global chemical cycles lies in recognizing the effects of such alterations.With the perception of an environmental crisis in the early 1970?s, more attention was paid to the role of human activity in these cycles.
Test lakes were studied to determine why freshwater fisheries were becoming oxygen-depleted at accelerated rates. Dramatic progress has been made in eliminating the problem of algal blooms and oxygen depletion by limiting the phosphorus-laden effluents being discharged into lakes.
- Nutrient Pollution
Nutrient pollution refers to a form of pollution in which nutrients, usually nitrogen and phosphorus, which are present in high concentrations is harmful to the ecosystem. Nutrient pollution is primarily a problem in aquatic ecosystems such as streams,...
- Calvin Cycle
Calvin cycleThe Calvin cycle is the principal mechanism that leads to the conversion of carbon dioxide into sugars by plants, algae, photosynthetic bacteria, and certain other bacteria that use chemicals as an energy source instead of light. The Calvin...
- Fertilizers
FertilizersFertilizers are materials used to modify the chemical composition of soil in order to enhance plant growth. They represent an important use of natural resources because agricultural systems depend upon an ability to retain soil fertility. Soil...
- Nutrient Cycling
Nutrient cycling Within an ecosystem, nutrients move through biogeochemical cycles. Those cycles involve chemical exchanges of elements among the earth?s atmosphere, water, living organisms, soil, and rocks. All biogeochemical cycles have a common structure,...
- Vegetables Nutrient Requirement For Specific Yields
Plant Nutrition is the study of the chemical elements and compounds that are necessary for plant growth, and also of their external supply and internal metabolism. In 1972, E. Epstein defined two criteria for an element to be essential for plant growth:In...
Agriculture
Phosphorus Cycle
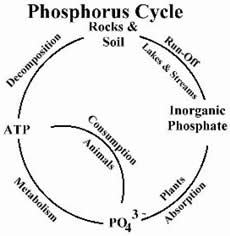 |
| Phosphorus Cycle |
The phosphorus cycle involves the movement of the element phosphorus as it circulates through the living and nonliving portions of the biosphere.
Many of the chemical elements found on the earth are vital to the processes and systems of living organisms. Unlike oxygen and carbon, phosphorus follows complex pathways. It circulates through the earth?s soils, rocks, waters, and atmosphere and through the organisms that inhabit these many ecosystems.
Elements or minerals are stored in discrete parts of the earth?s ecosystems called compartments. Examples of compartments include all the plants in a forest, a certain species of tree, or even the leaves or needles of a tree.
Chemical elements reside within the compartments in certain amounts, or pools. A basic description of biogeochemical cycles involves following nutrients in the form of minerals or elements from pool to pool through the multitudes of ecosystem compartments.
Phosphorus and Plants
Phosphorus compounds reside primarily in rocks. Phosphorus does not go through an atmospheric phase, but rather, phosphorus-laden rocks release phosphate (PO4?3) into the ecosystem as the result of weathering and erosion.
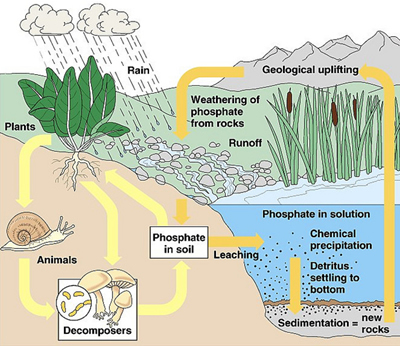 |
| Phosphorus and Plants |
To plants, phosphorus is a vital nutrient (second only to nitrogen). Plants absorb phosphates through their root hairs. Phosphorus then passes on through the food chain when the plants are consumed by other organisms.
Phosphorus is an essential component of many biological molecules, including deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Adenosine triphosphate (ATP), one of the nucleotides that make up DNA and RNA, is also the main energy transfer molecule in the multitude of chemical reactions taking place within organisms.
Because phosphorus is a major plant nutrient, massive amounts of phosphate-based fertilizers are either derived from natural sources (in the form of bat or bird guano) or chemically manufactured for use by agriculture.
As late as the early 1970?s, phosphates were a major constituent of household detergents, until it was discovered that large amounts of phosphates were being released into the environment.
 |
 |
In aquatic systems such as rivers and lakes, where such runoff eventually appears, an infusion of phosphates can cause algal blooms (rapidly forming, dense populations of algae). When the algae die, they are consumed by bacteria.
Decomposition by bacteria requires large amounts of oxygen, which soon depletes the available oxygen in the water. If the process is allowed to continue unchecked, fish and other organisms die from lack of oxygen. Both phosphates and nitrates contribute to cultural eutrophication.
Phosphates not taken up by plants go into the sedimentary phase, where they are very chemically reactive with other minerals. Some of these reactions produce compounds that effectively remove phosphates from the active nutrient pool.
This sedimentary phase is characterized by its long residence time compared to the rapid cycling through the biological phase. Phosphates can remain locked up in rocks for millions of years before being exposed and broken down by weathering, which once again makes them available to plants.
Phosphorus and the Environment
 |
| How to grow juicy tasty tomatoes |
Excessive phosphates in a eutrophic lake disrupt the carbon cycle by reacting with bicarbonates, thus increasing the pH. Many freshwater organisms depend on a neutral pH level for their survival.
The presence of phosphorus under these oxygen-depleted conditions can also indirectly affect the sulfur cycle, leading to the conversion of sulfate to sulfide. When sulfide combines with hydrogen to form the gas hydrogen sulfide, it takes on the familiar "rotten egg" smell.
One of the keys to preventing environmental degradation through the altering of global chemical cycles lies in recognizing the effects of such alterations.With the perception of an environmental crisis in the early 1970?s, more attention was paid to the role of human activity in these cycles.
Test lakes were studied to determine why freshwater fisheries were becoming oxygen-depleted at accelerated rates. Dramatic progress has been made in eliminating the problem of algal blooms and oxygen depletion by limiting the phosphorus-laden effluents being discharged into lakes.
- Nutrient Pollution
Nutrient pollution refers to a form of pollution in which nutrients, usually nitrogen and phosphorus, which are present in high concentrations is harmful to the ecosystem. Nutrient pollution is primarily a problem in aquatic ecosystems such as streams,...
- Calvin Cycle
Calvin cycleThe Calvin cycle is the principal mechanism that leads to the conversion of carbon dioxide into sugars by plants, algae, photosynthetic bacteria, and certain other bacteria that use chemicals as an energy source instead of light. The Calvin...
- Fertilizers
FertilizersFertilizers are materials used to modify the chemical composition of soil in order to enhance plant growth. They represent an important use of natural resources because agricultural systems depend upon an ability to retain soil fertility. Soil...
- Nutrient Cycling
Nutrient cycling Within an ecosystem, nutrients move through biogeochemical cycles. Those cycles involve chemical exchanges of elements among the earth?s atmosphere, water, living organisms, soil, and rocks. All biogeochemical cycles have a common structure,...
- Vegetables Nutrient Requirement For Specific Yields
Plant Nutrition is the study of the chemical elements and compounds that are necessary for plant growth, and also of their external supply and internal metabolism. In 1972, E. Epstein defined two criteria for an element to be essential for plant growth:In...


