Agriculture
Glycolysis is the beginning of the process of extracting usable energy from food. The disposal of the products of glycolysis when there is no oxygen available is the process of fermentation.
The simple sugar glucose is generally considered the starting point for looking at glycolysis and fermentation. Glucose is a simple carbohydrate, consisting of carbon, hydrogen, and oxygen.
Most glucose is produced by plants; organisms that cannot photosynthesize must obtain glucose (or more complex carbohydrates) from their surroundings. Animals obtain food molecules by eating. Simpler forms of life, such as bacteria and yeast, simply absorb their food from their environment.
Breaking Chemical Bonds
The energy in glucose is locked up in the chemical bonds that hold the molecule together. The process of glycolysis breaks these chemical bonds in a series of carefully controlled chemical reactions. Each reaction can be greatly accelerated by the appropriate enzyme.Generally, cells have sufficient quantities of the necessary enzymes present at all times.
Each chemical step is regulated by either the amount of raw materials present or the amount of finished product. If the raw materials are in short supply, the rate of reaction will be slow. Also, if the finished products build up to a high concentration, the reaction will slow down.
The energy of the chemical bonds in glucose must be released gradually. During most of the chemical steps, small amounts of energy are released. The amount of energy released is often not enough to perform significant biological work, in which case it is simply wasted as heat.
The energy released during some steps, however, is captured in the special high-energy bond of adenosine triphosphate (ATP). ATP is one of the most important of the short-term energy storage molecules in cells and is a coenzyme for many important chemical reactions.
Adenosine Triphosphate
ATP belongs to a class of organic molecules known as nucleotides. It has an important role in the energy reactions in the cell. The term ?triphosphate? indicates that there are three phosphate groups attached to the base molecule. The last two of these phosphates are held by a special kind of chemical bond known as a high-energy bond.
It takes a greater amount of energy to form one of these bonds than to form the normal kinds of bonds that hold the atoms of other molecules together. When this bond is broken, a large amount of energy is released and is available to the cell to do work.
Examples of such work are production of heat, synthesis of complex molecules, and movement of molecules across a membrane. When energy is required in a cell, the third phosphate of ATP is released. While the third phosphate group is routinely split off to release energy, the second one is rarely split off in cellular reactions.
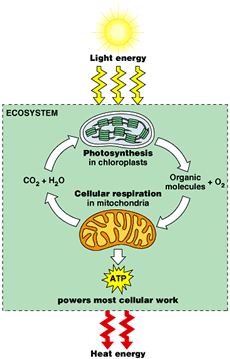
The cell must maintain a supply of ATP by means of the reverse reaction. The energy required for this reaction may come from fermentation when oxygen is unavailable. When oxygen is available, other components of cellular respiration are used, which include the Krebs cycle and electron transport.
Glycolysis
Energy from glycolysis is used to make ATP by two different processes. During glycolysis the glucose molecules are each split into two smaller molecules. The initial glucose molecules contain six carbon atoms each. Each molecule of glucose produces two molecules of pyruvic acid, and each pyruvic acidmolecule contains three carbon atoms.
During glycolysis, energy is released from the bonds of glucose molecules and is used to join free phosphate ions (also called inorganic phosphate or Pi) with molecules of adenosine diphosphate (ADP) to make ATP. This type of ATP synthesis is called substrate-level phosphorylation.
As a by-product, however, electrons are also stripped from glucose. These electrons are immediately trapped and held by another very important molecule, the electron carrier nicotinamide adenine dinucleotide (NAD).
By convention, the empty electron carrier is denoted as NAD+. When the molecule is carrying a pair of electrons, it is denoted as NADH, since the molecule also picks up a hydrogen nucleus, or proton. The electrons held by NADH represent potential energy.
In the presence of oxygen, these electrons can be passed to the electron transport system to make ADP by oxidative phosphorylation, while at the same time regenerating NAD+, which is required to maintain glycolysis. This second process for making ATP results in about eight times as much ATP per glucose molecule than from substrate-level phosphorylation in glycolysis.
Because fermentation is carried out in the absence of oxygen, this process cannot be used. Instead, the NADH must be relieved of its electrons by an alternative process. The NAD+ regeneration mechanism varies according to the type of organism.
Glucose molecules are relatively stable and do not split readily. For glucose molecules to split, they must be energized by the addition of two phosphate groups to each glucose molecule from two ATP molecules. The third phosphate from each ATP molecule is transferred, along with its high-energy bond.
Therefore, the initial steps of glycolysis actually use ATP, depleting some of the cell?s energy stores. Once glucose is energized, it readily splits under the influence of the appropriate enzyme. Each half of the glucose molecule then attaches another phosphate group from the cell?s pool of Pi.
In a series of reactions, each half of the glucose molecule generates two ATP molecules by substrate-level phosphorylation. Therefore, glycolysis results in a net gain of two molecules of ATP per molecule of glucose. At the end of glycolysis there are two three-carbon molecules of pyruvate left over for each original glucose molecule.
Fermentation
Under aerobic conditions, further energy from the chemical bonds of pyruvic acid is harvested by the Krebs cycle and electron transport system. When oxygen is not available (anaerobic conditions), however, the electrons must be removed from the NADH to regenerate NAD+.
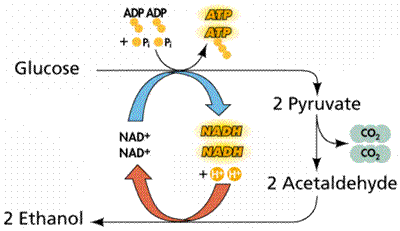
While there are many ways of accomplishing this, the most common methods are alcoholic fermentation, as observed in yeast, where the end products are ethyl alcohol and carbon dioxide, and lactic acid fermentation, as observed in the muscles of a mammal during strenuous physical exercise. In any event, no further energy is gained for the cell.
In yeast cells cultured in the absence of oxygen, a carbon atom and two oxygen atoms are first split from pyruvic acid, releasing a molecule of carbon dioxide (CO2). This CO2 gas is responsible for the bubbles that make bread rise and the carbonation in champagne.
The remainder of each pyruvic acid molecule then receives a pair of electrons from NADH, producing a molecule of ethyl alcohol (ethanol). The alcohol evaporates from bread when it is baked but is retained for its mildly euphoric effect in alcoholic beverages.
As far as the yeast is concerned, the alcohol is only produced as away of regenerating NAD+. It is not a desirable product and will eventually kill the yeast cells. Most yeast cells cannot tolerate an alcohol concentration greater than about 12 percent.
Cellular respiration is the process by which organisms harvest usable energy in the form of ATP molecules from food molecules. Fermentation is the form of respiration used when oxygen is not available.
Fermentation is much less efficient than aerobic cellular respiration. Fermentation harvests only two molecules of ATP for every glucose molecule used. Aerobic respiration reaps a yield of more than thirty molecules of ATP.
Additionally, the typical products of fermentation, alcohol or lactic acid, are toxic to the organism producing them. Most forms of life will resort to fermentation only when oxygen is absent or in short supply. These are described as facultative anaerobes.
While higher forms of life, such as animals, can obtain energy by fermentation for short periods, they enter an oxygen debt, which must eventually be repaid. The yield of two molecules of ATP for each glucose molecule used is simply not enough to sustain their high demand for energy.
A few simple forms of life, mostly bacteria, rely solely on fermentation for their source of ATP. To some of these, oxygen is actually poisonous. These are described as obligate anaerobes, and they are only found under the completely anaerobic conditions of the deeper layers of mud in saltwater and freshwater marshes.
- Anaerobes And Heterotrophs
AnaerobesThe first organisms to evolve on the earth are thought to have been heterotrophs and anerobes. Heterotrophs are organisms that cannot produce their own food but must fill their energy requirements by consuming organic molecules produced by other...
- Carbohydrates
Common organic chemicals found in all living organisms, important in energy metabolism and structural polymers, carbohydrate molecules are made up of carbon, hydrogen, and oxygen. Carbohydrates are made of carbon, hydrogen, and oxygen molecules in a...
- Energy Flow In Plant Cells
Energy flow in plant cells Life on earth is dependent on the flow of energy from the sun. A small portion of the solar energy, captured in the process of photosynthesis, drives many chemical reactions associated with living systems. In living organisms,...
- Exergonic And Endergonic Reactions
Exergonic ReactionsExergonic reactions are spontaneous chemical reactions in which the products are at a lower energy level than the reactants; these reactions release energy. Endergonic reactions are nonspontaneous chemical reactions in which the products...
- Oxidative Phosphorylation
Oxidative Phosphorylation Oxidative phosphorylation is the sequence of reactions in mitochondria that convert energy from food into cellular energy by synthesizing ATP, the primary energy currency of cells. To drive the second step in oxidative phosphorylation,...
Agriculture
Glycolysis and Fermentation
 |
| Glycolysis |
The simple sugar glucose is generally considered the starting point for looking at glycolysis and fermentation. Glucose is a simple carbohydrate, consisting of carbon, hydrogen, and oxygen.
Most glucose is produced by plants; organisms that cannot photosynthesize must obtain glucose (or more complex carbohydrates) from their surroundings. Animals obtain food molecules by eating. Simpler forms of life, such as bacteria and yeast, simply absorb their food from their environment.
Breaking Chemical Bonds
The energy in glucose is locked up in the chemical bonds that hold the molecule together. The process of glycolysis breaks these chemical bonds in a series of carefully controlled chemical reactions. Each reaction can be greatly accelerated by the appropriate enzyme.Generally, cells have sufficient quantities of the necessary enzymes present at all times.
Each chemical step is regulated by either the amount of raw materials present or the amount of finished product. If the raw materials are in short supply, the rate of reaction will be slow. Also, if the finished products build up to a high concentration, the reaction will slow down.
The energy of the chemical bonds in glucose must be released gradually. During most of the chemical steps, small amounts of energy are released. The amount of energy released is often not enough to perform significant biological work, in which case it is simply wasted as heat.
The energy released during some steps, however, is captured in the special high-energy bond of adenosine triphosphate (ATP). ATP is one of the most important of the short-term energy storage molecules in cells and is a coenzyme for many important chemical reactions.
Adenosine Triphosphate
 |
 |
ATP belongs to a class of organic molecules known as nucleotides. It has an important role in the energy reactions in the cell. The term ?triphosphate? indicates that there are three phosphate groups attached to the base molecule. The last two of these phosphates are held by a special kind of chemical bond known as a high-energy bond.
It takes a greater amount of energy to form one of these bonds than to form the normal kinds of bonds that hold the atoms of other molecules together. When this bond is broken, a large amount of energy is released and is available to the cell to do work.
Examples of such work are production of heat, synthesis of complex molecules, and movement of molecules across a membrane. When energy is required in a cell, the third phosphate of ATP is released. While the third phosphate group is routinely split off to release energy, the second one is rarely split off in cellular reactions.
The cell must maintain a supply of ATP by means of the reverse reaction. The energy required for this reaction may come from fermentation when oxygen is unavailable. When oxygen is available, other components of cellular respiration are used, which include the Krebs cycle and electron transport.
Glycolysis
Energy from glycolysis is used to make ATP by two different processes. During glycolysis the glucose molecules are each split into two smaller molecules. The initial glucose molecules contain six carbon atoms each. Each molecule of glucose produces two molecules of pyruvic acid, and each pyruvic acidmolecule contains three carbon atoms.
During glycolysis, energy is released from the bonds of glucose molecules and is used to join free phosphate ions (also called inorganic phosphate or Pi) with molecules of adenosine diphosphate (ADP) to make ATP. This type of ATP synthesis is called substrate-level phosphorylation.
As a by-product, however, electrons are also stripped from glucose. These electrons are immediately trapped and held by another very important molecule, the electron carrier nicotinamide adenine dinucleotide (NAD).
By convention, the empty electron carrier is denoted as NAD+. When the molecule is carrying a pair of electrons, it is denoted as NADH, since the molecule also picks up a hydrogen nucleus, or proton. The electrons held by NADH represent potential energy.
In the presence of oxygen, these electrons can be passed to the electron transport system to make ADP by oxidative phosphorylation, while at the same time regenerating NAD+, which is required to maintain glycolysis. This second process for making ATP results in about eight times as much ATP per glucose molecule than from substrate-level phosphorylation in glycolysis.
Because fermentation is carried out in the absence of oxygen, this process cannot be used. Instead, the NADH must be relieved of its electrons by an alternative process. The NAD+ regeneration mechanism varies according to the type of organism.
Glucose molecules are relatively stable and do not split readily. For glucose molecules to split, they must be energized by the addition of two phosphate groups to each glucose molecule from two ATP molecules. The third phosphate from each ATP molecule is transferred, along with its high-energy bond.
Therefore, the initial steps of glycolysis actually use ATP, depleting some of the cell?s energy stores. Once glucose is energized, it readily splits under the influence of the appropriate enzyme. Each half of the glucose molecule then attaches another phosphate group from the cell?s pool of Pi.
In a series of reactions, each half of the glucose molecule generates two ATP molecules by substrate-level phosphorylation. Therefore, glycolysis results in a net gain of two molecules of ATP per molecule of glucose. At the end of glycolysis there are two three-carbon molecules of pyruvate left over for each original glucose molecule.
Fermentation
 |
| Fermentation |
Under aerobic conditions, further energy from the chemical bonds of pyruvic acid is harvested by the Krebs cycle and electron transport system. When oxygen is not available (anaerobic conditions), however, the electrons must be removed from the NADH to regenerate NAD+.
While there are many ways of accomplishing this, the most common methods are alcoholic fermentation, as observed in yeast, where the end products are ethyl alcohol and carbon dioxide, and lactic acid fermentation, as observed in the muscles of a mammal during strenuous physical exercise. In any event, no further energy is gained for the cell.
In yeast cells cultured in the absence of oxygen, a carbon atom and two oxygen atoms are first split from pyruvic acid, releasing a molecule of carbon dioxide (CO2). This CO2 gas is responsible for the bubbles that make bread rise and the carbonation in champagne.
The remainder of each pyruvic acid molecule then receives a pair of electrons from NADH, producing a molecule of ethyl alcohol (ethanol). The alcohol evaporates from bread when it is baked but is retained for its mildly euphoric effect in alcoholic beverages.
As far as the yeast is concerned, the alcohol is only produced as away of regenerating NAD+. It is not a desirable product and will eventually kill the yeast cells. Most yeast cells cannot tolerate an alcohol concentration greater than about 12 percent.
Cellular respiration is the process by which organisms harvest usable energy in the form of ATP molecules from food molecules. Fermentation is the form of respiration used when oxygen is not available.
Fermentation is much less efficient than aerobic cellular respiration. Fermentation harvests only two molecules of ATP for every glucose molecule used. Aerobic respiration reaps a yield of more than thirty molecules of ATP.
 |
| wine making made easy |
Additionally, the typical products of fermentation, alcohol or lactic acid, are toxic to the organism producing them. Most forms of life will resort to fermentation only when oxygen is absent or in short supply. These are described as facultative anaerobes.
While higher forms of life, such as animals, can obtain energy by fermentation for short periods, they enter an oxygen debt, which must eventually be repaid. The yield of two molecules of ATP for each glucose molecule used is simply not enough to sustain their high demand for energy.
A few simple forms of life, mostly bacteria, rely solely on fermentation for their source of ATP. To some of these, oxygen is actually poisonous. These are described as obligate anaerobes, and they are only found under the completely anaerobic conditions of the deeper layers of mud in saltwater and freshwater marshes.
- Anaerobes And Heterotrophs
AnaerobesThe first organisms to evolve on the earth are thought to have been heterotrophs and anerobes. Heterotrophs are organisms that cannot produce their own food but must fill their energy requirements by consuming organic molecules produced by other...
- Carbohydrates
Common organic chemicals found in all living organisms, important in energy metabolism and structural polymers, carbohydrate molecules are made up of carbon, hydrogen, and oxygen. Carbohydrates are made of carbon, hydrogen, and oxygen molecules in a...
- Energy Flow In Plant Cells
Energy flow in plant cells Life on earth is dependent on the flow of energy from the sun. A small portion of the solar energy, captured in the process of photosynthesis, drives many chemical reactions associated with living systems. In living organisms,...
- Exergonic And Endergonic Reactions
Exergonic ReactionsExergonic reactions are spontaneous chemical reactions in which the products are at a lower energy level than the reactants; these reactions release energy. Endergonic reactions are nonspontaneous chemical reactions in which the products...
- Oxidative Phosphorylation
Oxidative Phosphorylation Oxidative phosphorylation is the sequence of reactions in mitochondria that convert energy from food into cellular energy by synthesizing ATP, the primary energy currency of cells. To drive the second step in oxidative phosphorylation,...


