Agriculture
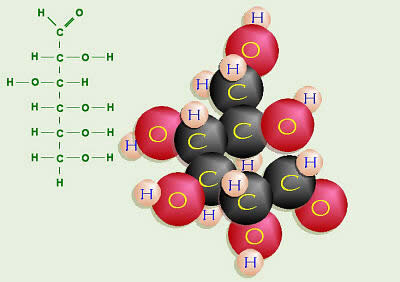
Common organic chemicals found in all living organisms, important in energy metabolism and structural polymers, carbohydrate molecules are made up of carbon, hydrogen, and oxygen.
Carbohydrates are made of carbon, hydrogen, and oxygen molecules in a 1:2:1 ratio, respectively. This is often simplified using the formula nCH2O, where n represents the number of CH2O subunits in a carbohydrate. This formula should make it clear how the name carbohydrate was derived, as nCH2O is essentially carbon and water.
The simplest carbohydrates are the monosaccharides, or simple sugars. Individual monosaccharides can be joined together to make disaccharides (composed of two monosaccharides), oligosaccharides (short polymers composed of two to several monosaccharides), and polysaccharides (longer polymers composed of numerous monosaccharides).
Monosaccharides
The common monosaccharides found in plants have from three to six carbon atoms in a straight chain with one oxygen atom. Most of the oxygen atoms also have a hydrogen atom attached, making them hydroxyl groups (?OH). One of the oxygen atoms is connected to a carbon by a double covalent bond, while the hydroxyl groups are attached to carbon atoms by single covalent bonds.
If the double-bonded oxygen is on a terminal carbon (as an aldehyde group), the monosaccharide is called an aldose. If the double-bonded oxygen is on an internal carbon, the monosaccharide is called a ketose.
The simplest monosaccharides are the three carbon sugars, or trioses. Pentoses, with five carbons, are also important in plants.
Ribose and deoxyribose are found in RNA(ribonucleic acid) and DNA(deoxyribonucleic acid), respectively. Ribulose bisphosphate is an important intermediate in the incorporation of carbon dioxide into carbohydrates during photosynthesis. Xylose and arabinose are found as components of some plant polysaccharides.
Hexoses, six-carbon monosaccharides such as glucose, fructose, and galactose, are the most common monosaccharides in plants. These sugars all have the same formula, C6H12O6 (note the 1:2:1 ratio of C:H:O), but their atoms are arranged differently. Glucose is the primary carbon-containing product of photosynthesis and reverse glycolysis and later can be metabolized through glycolysis and the Krebs cycle to release energy or can be converted to other carbohydrates needed by the plant.
Oligosaccharides
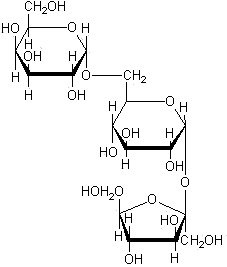 Oligosaccharides are made by joining two or more monosaccharides. The smallest are the disaccharides, formed from two monosaccharides that are joined together by a condensation reaction. Condensation reactions get their name from the fact that when the two monosaccharides are joined together, a molecule of water is released.
Oligosaccharides are made by joining two or more monosaccharides. The smallest are the disaccharides, formed from two monosaccharides that are joined together by a condensation reaction. Condensation reactions get their name from the fact that when the two monosaccharides are joined together, a molecule of water is released.
Sucrose (glucose-fructose) is the most common plant disaccharide and is the principal molecule of short-term energy storage and of translocation (transport) in the phloem. Many plants, including sugarcane (Saccharum officinarum) and sugar beets (Beta saccharifera), have high concentrations of sucrose, which can be extracted and refined for use as table sugar.
Other disaccharides found in plants are maltose, which is a glucose disaccharide formed from the hydrolysis (the reverse of a condensation reaction, wherein water is used to ?split? the bond between the monosaccharides) of starch, and trehalose, also a glucose disaccharide, which is the primary molecule of translocation in species of Selaginella and is seen in cyanobacteria (blue-green algae or blue-green bacteria), red algae, and fungi. Cellobiose, another glucose disaccharide, is formed by the hydrolysis of cellulose.
The trisaccharide raffinose (galactose-glucose-fructose) is a storage molecule in sugar beets and in cotton and legume seeds. Stachyose (galactose-galactose-glucose-fructose) and verbascose (galactose-galactose-galactose-glucose-fructose) are also storage oligosaccharides, seen mainly in Fabaceae (the legume or pea family).
Polysaccharides
The two main functions of polysaccharides in plants are long-term energy storage and structure. Glucose is the most common subunit in plant polysaccharides. The glucose molecules in these polymers are joined together in different ways. The carbon atoms in glucose molecules are numbered from one to six.
In some, the 1-carbon of a glucose is attached to the 4-carbon of the next, and this linkage is repeated throughout the molecule. At other times, an additional bond is formed between the 1-carbon and the 6-carbon of adjacent glucoses, which results in a branched polysaccharide.
Starch is the most common storage polysaccharide of plants. Two forms of this glucose polymer exist. Amylose is a linear polymer made up of between one hundred and several thousand glucose units. Amylopectin is very similar, but it is a branched polymer.
In most plants, starch is 15-25 percent amylose and 75-85 percent amylopectin. However, starch in some waxy varieties of corn is nearly 100 percent amylopectin and in some wrinkled varieties of peas is as high as 80 percent amylose. Phytoglycogen found on corn (Zea mays) is an even more branched glucose polymer.
Fructosans are another type of storage polysaccharide in plants. They are branched or unbranched fructose polymers with a terminal glucose subunit. Inulin is found in the tubers or rhizomes of plants in Campanulaceae (the bellflower family) and Asteraceae (the sunflower or aster family) and usually has thirty to fifty fructose subunits.
Levans, used for temporary storage by several monocots, especially in Poaceae (the grass family), range from seven to eight fructose subunits in the unbranched levans to seventy-two fructose subunits in some highly branched ones.
Structural Polysaccharides
 Structural polysaccharides form the fibrous material in plant cell walls. Cellulose, an unbranched glucose polymer that averages about eight thousand glucose subunits per molecule, is the main cell wall component of plants, a few fungi, and some algae. Cellulose molecules form microfibrils, many individual cellulose molecules held together by hydrogen bonds. Other microfibrillar cell wall polysaccharides are sometimes called the hemicelluloses.
Structural polysaccharides form the fibrous material in plant cell walls. Cellulose, an unbranched glucose polymer that averages about eight thousand glucose subunits per molecule, is the main cell wall component of plants, a few fungi, and some algae. Cellulose molecules form microfibrils, many individual cellulose molecules held together by hydrogen bonds. Other microfibrillar cell wall polysaccharides are sometimes called the hemicelluloses.
Examples are mannans and glucomannans, found in the primary cell walls of several green algae and simple vascular plants and in the secondary cell walls of some conifers; xylans are found in other algae and in the secondary cell walls of many hardwoods. Chitin, a polymer of N-acetylglucosamine, is the main substance forming the cell walls of fungi. (Chitin is the same substance that forms the exoskeletons of most insects.)
Pectins are matrix polysaccharides found in plant cell walls. The most common pectin in higher plants is unbranched polygalacturonic acid (galacturan). Branched and unbranched rhamnogalacturans and arabinans are also present in smaller quantities.
Pectin is commercially important as a gelling agent in the production of jams and jellies. A similar pectin like polysaccharide found in brown algae is alginic acid, a mixture of mannuronic and guluronic acids. It is used as a thickener and a stabilizer in many prepared foods.
Other Plant Carbohydrates
Carbohydrates are often found attached to other cell components. In both cell membranes and cell walls, there are many glycoproteins, proteins with short oligosaccharides attached.
Glycosides are interesting carbohydrate-containing secondary metabolites found in many plants. Glycosides are formed when carbohydrates are attached to various plant chemicals. Anthocyanins, which give red to blue color to flowers, fruits, and autumn leaves, are glycosides.
Other glycosides include the cardiac glycosides of the foxglove (Digitalis purpurea) and milkweed (Asclepias) species, which have strong physiological effects on heart muscle, and the cyanogenic glycosides of the almond (Prunus amygdalus), which liberate cyanide.
- Calvin Cycle
Calvin cycleThe Calvin cycle is the principal mechanism that leads to the conversion of carbon dioxide into sugars by plants, algae, photosynthetic bacteria, and certain other bacteria that use chemicals as an energy source instead of light. The Calvin...
- Cell Wall
Cell WallThe cell wall is the outer, rigid wall of a cell, dividing the protoplast (the interior, including the cytoplasm and nucleus) from the cell?s external environment. The plant cell wall is both unique to and a major feature of plants, perhaps second...
- Exergonic And Endergonic Reactions
Exergonic ReactionsExergonic reactions are spontaneous chemical reactions in which the products are at a lower energy level than the reactants; these reactions release energy. Endergonic reactions are nonspontaneous chemical reactions in which the products...
- Glycolysis And Fermentation
GlycolysisGlycolysis is the beginning of the process of extracting usable energy from food. The disposal of the products of glycolysis when there is no oxygen available is the process of fermentation. The simple sugar glucose is generally considered the...
- Respiration
RespirationAll cells must have a source of energy in order to survive. Almost all cells utilize ATP as their energy currency. In other words, ATP is produced and stored up until it is needed to supply energy for metabolic activity. Respiration is the...
Agriculture
Carbohydrates

Common organic chemicals found in all living organisms, important in energy metabolism and structural polymers, carbohydrate molecules are made up of carbon, hydrogen, and oxygen.
Carbohydrates are made of carbon, hydrogen, and oxygen molecules in a 1:2:1 ratio, respectively. This is often simplified using the formula nCH2O, where n represents the number of CH2O subunits in a carbohydrate. This formula should make it clear how the name carbohydrate was derived, as nCH2O is essentially carbon and water.
The simplest carbohydrates are the monosaccharides, or simple sugars. Individual monosaccharides can be joined together to make disaccharides (composed of two monosaccharides), oligosaccharides (short polymers composed of two to several monosaccharides), and polysaccharides (longer polymers composed of numerous monosaccharides).
Monosaccharides
The common monosaccharides found in plants have from three to six carbon atoms in a straight chain with one oxygen atom. Most of the oxygen atoms also have a hydrogen atom attached, making them hydroxyl groups (?OH). One of the oxygen atoms is connected to a carbon by a double covalent bond, while the hydroxyl groups are attached to carbon atoms by single covalent bonds.
If the double-bonded oxygen is on a terminal carbon (as an aldehyde group), the monosaccharide is called an aldose. If the double-bonded oxygen is on an internal carbon, the monosaccharide is called a ketose.
The simplest monosaccharides are the three carbon sugars, or trioses. Pentoses, with five carbons, are also important in plants.
Ribose and deoxyribose are found in RNA(ribonucleic acid) and DNA(deoxyribonucleic acid), respectively. Ribulose bisphosphate is an important intermediate in the incorporation of carbon dioxide into carbohydrates during photosynthesis. Xylose and arabinose are found as components of some plant polysaccharides.
Hexoses, six-carbon monosaccharides such as glucose, fructose, and galactose, are the most common monosaccharides in plants. These sugars all have the same formula, C6H12O6 (note the 1:2:1 ratio of C:H:O), but their atoms are arranged differently. Glucose is the primary carbon-containing product of photosynthesis and reverse glycolysis and later can be metabolized through glycolysis and the Krebs cycle to release energy or can be converted to other carbohydrates needed by the plant.
Oligosaccharides

Sucrose (glucose-fructose) is the most common plant disaccharide and is the principal molecule of short-term energy storage and of translocation (transport) in the phloem. Many plants, including sugarcane (Saccharum officinarum) and sugar beets (Beta saccharifera), have high concentrations of sucrose, which can be extracted and refined for use as table sugar.
Other disaccharides found in plants are maltose, which is a glucose disaccharide formed from the hydrolysis (the reverse of a condensation reaction, wherein water is used to ?split? the bond between the monosaccharides) of starch, and trehalose, also a glucose disaccharide, which is the primary molecule of translocation in species of Selaginella and is seen in cyanobacteria (blue-green algae or blue-green bacteria), red algae, and fungi. Cellobiose, another glucose disaccharide, is formed by the hydrolysis of cellulose.
The trisaccharide raffinose (galactose-glucose-fructose) is a storage molecule in sugar beets and in cotton and legume seeds. Stachyose (galactose-galactose-glucose-fructose) and verbascose (galactose-galactose-galactose-glucose-fructose) are also storage oligosaccharides, seen mainly in Fabaceae (the legume or pea family).
Polysaccharides
The two main functions of polysaccharides in plants are long-term energy storage and structure. Glucose is the most common subunit in plant polysaccharides. The glucose molecules in these polymers are joined together in different ways. The carbon atoms in glucose molecules are numbered from one to six.
In some, the 1-carbon of a glucose is attached to the 4-carbon of the next, and this linkage is repeated throughout the molecule. At other times, an additional bond is formed between the 1-carbon and the 6-carbon of adjacent glucoses, which results in a branched polysaccharide.
Starch is the most common storage polysaccharide of plants. Two forms of this glucose polymer exist. Amylose is a linear polymer made up of between one hundred and several thousand glucose units. Amylopectin is very similar, but it is a branched polymer.
In most plants, starch is 15-25 percent amylose and 75-85 percent amylopectin. However, starch in some waxy varieties of corn is nearly 100 percent amylopectin and in some wrinkled varieties of peas is as high as 80 percent amylose. Phytoglycogen found on corn (Zea mays) is an even more branched glucose polymer.
Fructosans are another type of storage polysaccharide in plants. They are branched or unbranched fructose polymers with a terminal glucose subunit. Inulin is found in the tubers or rhizomes of plants in Campanulaceae (the bellflower family) and Asteraceae (the sunflower or aster family) and usually has thirty to fifty fructose subunits.
Levans, used for temporary storage by several monocots, especially in Poaceae (the grass family), range from seven to eight fructose subunits in the unbranched levans to seventy-two fructose subunits in some highly branched ones.
Structural Polysaccharides

Examples are mannans and glucomannans, found in the primary cell walls of several green algae and simple vascular plants and in the secondary cell walls of some conifers; xylans are found in other algae and in the secondary cell walls of many hardwoods. Chitin, a polymer of N-acetylglucosamine, is the main substance forming the cell walls of fungi. (Chitin is the same substance that forms the exoskeletons of most insects.)
Pectins are matrix polysaccharides found in plant cell walls. The most common pectin in higher plants is unbranched polygalacturonic acid (galacturan). Branched and unbranched rhamnogalacturans and arabinans are also present in smaller quantities.
Pectin is commercially important as a gelling agent in the production of jams and jellies. A similar pectin like polysaccharide found in brown algae is alginic acid, a mixture of mannuronic and guluronic acids. It is used as a thickener and a stabilizer in many prepared foods.
Other Plant Carbohydrates
Carbohydrates are often found attached to other cell components. In both cell membranes and cell walls, there are many glycoproteins, proteins with short oligosaccharides attached.
Glycosides are interesting carbohydrate-containing secondary metabolites found in many plants. Glycosides are formed when carbohydrates are attached to various plant chemicals. Anthocyanins, which give red to blue color to flowers, fruits, and autumn leaves, are glycosides.
Other glycosides include the cardiac glycosides of the foxglove (Digitalis purpurea) and milkweed (Asclepias) species, which have strong physiological effects on heart muscle, and the cyanogenic glycosides of the almond (Prunus amygdalus), which liberate cyanide.
- Calvin Cycle
Calvin cycleThe Calvin cycle is the principal mechanism that leads to the conversion of carbon dioxide into sugars by plants, algae, photosynthetic bacteria, and certain other bacteria that use chemicals as an energy source instead of light. The Calvin...
- Cell Wall
Cell WallThe cell wall is the outer, rigid wall of a cell, dividing the protoplast (the interior, including the cytoplasm and nucleus) from the cell?s external environment. The plant cell wall is both unique to and a major feature of plants, perhaps second...
- Exergonic And Endergonic Reactions
Exergonic ReactionsExergonic reactions are spontaneous chemical reactions in which the products are at a lower energy level than the reactants; these reactions release energy. Endergonic reactions are nonspontaneous chemical reactions in which the products...
- Glycolysis And Fermentation
GlycolysisGlycolysis is the beginning of the process of extracting usable energy from food. The disposal of the products of glycolysis when there is no oxygen available is the process of fermentation. The simple sugar glucose is generally considered the...
- Respiration
RespirationAll cells must have a source of energy in order to survive. Almost all cells utilize ATP as their energy currency. In other words, ATP is produced and stored up until it is needed to supply energy for metabolic activity. Respiration is the...
